Significant improvement in daily function
and muscle weakness†
*As measured by the Myasthenia Gravis Activities of Daily Living (MG-ADL) scale.
†As measured by the Quantitative Myasthenia Gravis (QMG) scale.
A 24-week clinical study explored the effectiveness and safety of IMAAVY in adult patients living with antibody-positive gMG (including those with anti-AChR or anti-MuSK gMG).
4+
point reduction in
MG-ADL on average
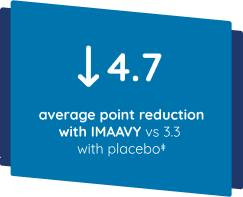
Patients taking IMAAVY + current gMG treatment experienced a 4.7-point improvement in MG-ADL on average. Patients taking placebo + current gMG treatment experienced a 3.3-point improvement on average. This was measured using results averaged from Weeks 22, 23, and 24 of the study compared to baseline.
What could an improvement in MG-ADL total score mean?
The MG-ADL scale assesses the impact of gMG on 8 daily-function items (talking, chewing, swallowing, breathing, brushing teeth or hair, rising from a chair, double vision, and eyelid droop) that are typically affected in gMG. A lower MG-ADL score can indicate improvement in the ability to perform these 8 activities of daily living.
Individual results may vary.
Talk to your healthcare provider to see if IMAAVY is right for you.
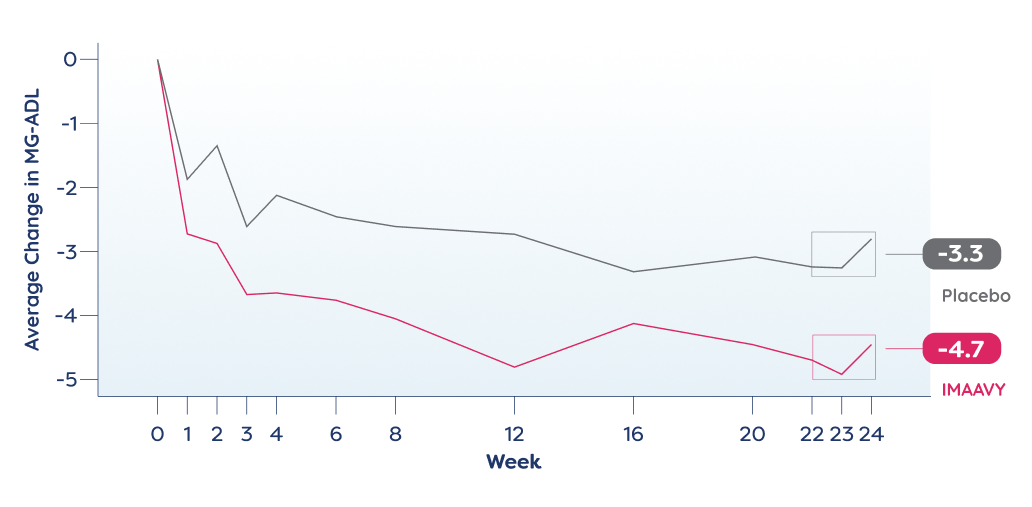


IMAAVY doses were given once every 2 weeks after a starter dose.
Patients received either IMAAVY + current gMG treatment or placebo + current gMG treatment.
‡This was measured using results averaged from Weeks 22, 23, and 24 of the study compared to baseline.
4+
point reduction in
QMG on average
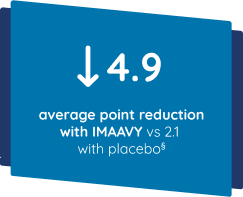
Patients taking IMAAVY + current gMG treatment experienced a 4.9-point improvement in QMG on average. Patients taking placebo + current gMG treatment experienced a 2.1-point improvement on average. This was measured using results averaged from Weeks 22 and 24 of the study compared to baseline.
What could an improvement in QMG total score mean?
The QMG scale assesses muscle weakness based on 13 items. A lower QMG score can indicate improvement in muscle weakness or less muscle weakness.
Individual results may vary. Talk to your healthcare provider to see if IMAAVY is right for you.

IMAAVY doses were given once every 2 weeks after a starter dose.
Patients received either IMAAVY + current gMG treatment or placebo + current gMG treatment.
§This was measured using results averaged from Weeks 22 and 24 of the study compared to baseline.


IMAAVY doses were given once every 2 weeks after a starter dose.
Patients received either IMAAVY + current gMG treatment or placebo + current gMG treatment.
§This was measured using results averaged from Weeks 22 and 24 of the study compared to baseline.
IMAAVY plus current gMG treatment compared to placebo plus current gMG treatment was studied in 153 adults, which included anti-AChR or anti-MuSK antibody-positive gMG patients.
The safety was evaluated across the full population.
The effectiveness of IMAAVY was measured using the average change in daily function (MG-ADL)‖ and muscle weakness (QMG)¶ scores. Patients received either IMAAVY + their current gMG treatment or placebo + their current gMG treatment once every 2 weeks.
This study required patients to meet the following criteria:
The MG-ADL is a scale that measures your ability to perform 8 daily tasks typically affected by your gMG. Some items in the scale include talking, brushing your teeth, and chewing.
The QMG is a 13-item scale that measures muscle weakness. Some items in the scale include arm strength, leg strength, and grip strength.
||This was measured using results averaged from Weeks 22, 23, and 24 of the study compared to baseline.
¶This was measured using results averaged from Weeks 22 and 24 of the study compared to baseline.
The MG-ADL scale assesses the impact of gMG on 8 daily-function items that are typically affected in gMG, based on information that you provide to your healthcare provider.
Talking
Swallowing
Brushing teeth or hair
Double vision
Chewing
Breathing
Rising from chair
Eyelid droop
The MG-ADL is scored on a scale of 0–3 in each symptom category.
You might think of your MG-ADL score like a golf score or other game in which the lowest number is preferred. A low MG-ADL score means your gMG symptoms are less severe.
The QMG scale assesses muscle weakness based on 13 items. Your healthcare provider will take you through the QMG evaluation and record the results.
Double vision and upper eyelid droop
Ability to drink water
Counting out loud
Grip strength
Face and neck
muscle strength
Breathing capacity
Arm strength
Leg strength
Like the MG-ADL, the QMG is also scored on a scale of 0–3 in each symptom category.
Like the MG-ADL scale, lower numbers on the QMG mean a better score. However, your healthcare provider records those scores differently. You might think of it like a fitness exam in which your healthcare provider is testing to see how well you can perform each task.


These are not all of the possible side effects of IMAAVY.
If you have a reaction during your IMAAVY infusion, your healthcare provider may decide to give IMAAVY more slowly or to stop your infusion.
Please read the Important Safety Information and the Medication Guide for IMAAVY to learn more about these and other risks for IMAAVY. Discuss any questions you have with your healthcare provider.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Pediatric patients ages 12 to 16
years who were anti-AChR+
Adults who were anti-AChR+ or
anti-MuSK+ including women of
childbearing age
In gMG, sometimes there are harmful immunoglobulin G (IgG) antibodies such as harmful anti-AChR and anti-MuSK antibodies that can target receptors in your muscles, preventing those muscles from working properly. This is what could cause gMG symptoms, as your nerves and muscles are communicating less effectively.
Neonatal fragment crystallizable receptor (FcRn) is a protein in your body that can help keep harmful IgG antibodies circulating in your body longer, where they can continue to attach to muscle receptors and interfere with signals sent from nerves.
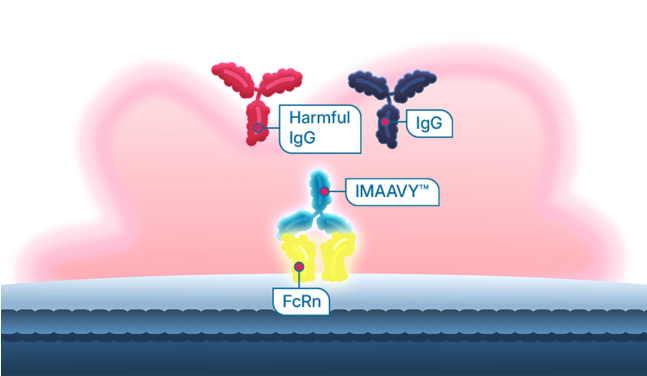
Because IMAAVY binds and blocks FcRn receptors, harmful IgG antibodies, including anti-AChR and anti-MuSK, are reduced.
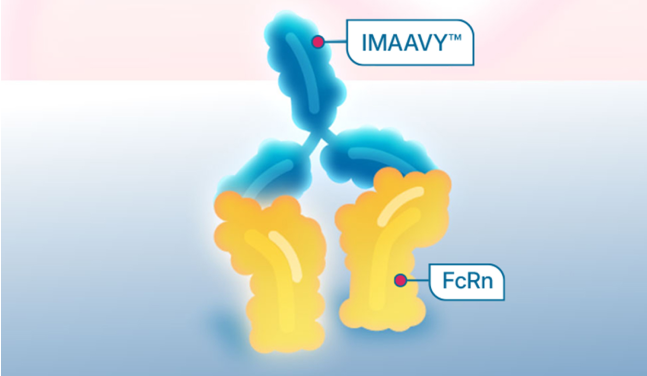
IMAAVY binds and blocks FcRn.
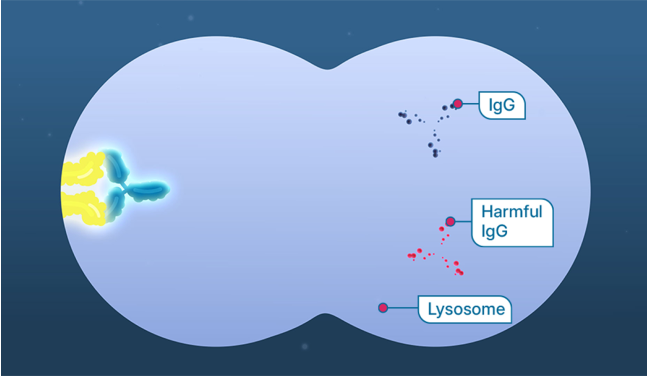
This can help prevent IgG, including harmful IgG antibodies, from binding to FcRn, which leads to the breakdown of IgG antibodies.
IgG antibody levels are reduced, including the harmful anti-AChR and anti-MuSK antibodies. These harmful antibodies can cause gMG symptoms.
Hello. My name is Kimberly, and I’ve been living with generalized myasthenia gravis, or gMG, for several years. Today, I want to talk to you about how gMG affects your body. And later, we will talk about a treatment designed to help reduce the harmful antibodies that cause gMG symptoms by blocking FcRn receptors. If you’re living with gMG, you know it’s an autoimmune condition that causes muscle weakness, sometimes making even simple tasks feel challenging.
All of our bodies produce a protein known as an IgG, or immunoglobulin antibody. These antibodies protect us from outside viruses and bacteria that can make us sick. But in a person living with gMG, the body may also create harmful IgG antibodies. From here on, we’ll refer to them as “harmful IgG.” These antibodies can mistakenly target the receptors on your muscles, interrupting the nerve signals that tell your muscles to move.
When signals between your nerves and muscles are interrupted, it can cause gMG symptoms. These can include muscle weakness and muscle fatigue, which can make everyday tasks more difficult. To really understand how gMG impacts the body, let’s explore what’s happening on the inside, together.
Great, you’re here! Now, let’s take a closer look at what’s happening inside people living with gMG. As I mentioned earlier, the normal function of IgG is to help the body fight off invading viruses and bacteria. But in gMG, there can be harmful IgG that target receptors at a place called the neuromuscular junction – the site where nerves and muscles meet.
At the neuromuscular junction, nerve cells send signals to muscle cells. These signals act as instructions that help tell muscles to move. The signals are received by the receptors on muscle cells. Harmful IgG can attach to and block those important receptors, preventing signals from reaching your muscles to tell them to move. This could result in gMG symptoms. The main receptor that receives the signals from the nerves is called the acetylcholine receptor, or AChR. People with harmful IgG that target this receptor are known to have anti-AChR antibody-positive gMG.
Another receptor involved at the neuromuscular junction is the MuSK receptor. It’s less common for people living with gMG to have harmful IgG that target MuSK. People with harmful IgG that target this receptor are known to have anti-MuSK antibody-positive gMG. While harmful IgG are key players in gMG, another receptor is equally important to understand. It’s called the neonatal fragment crystallizable receptor – also known as F-C-R-N – and it controls how long IgG stays in the body.
FcRn is a protein in your body that is responsible for recycling IgG, and it increases how long IgG stays within the body. Part of the lifecycle of IgG includes a process in which they are broken down, or degraded, inside the cell.
FcRn protects IgG from being degraded and recycles them back into the body. However, FcRn doesn’t have the ability to recognize the difference between helpful and harmful IgG. This extends the life of IgG, including harmful IgG, meaning both helpful and harmful IgG stay in your body longer.
When harmful IgG are recycled and kept in your body, they can continue to interfere with the communication between your nerves and muscles. This can continue to lead to gMG symptoms. We’ve now got a good idea of what’s happening inside the body when it comes to gMG disease activity. Now, let’s learn about a treatment that might be able to help.
Still with me? Great. It’s time to learn about a type of treatment that disrupts the binding of IgG to FcRn. This treatment is called an FcRn blocker. How does it work? It binds to FcRn, which can help prevent some IgG from binding to FcRn. IgG that do not bind to FcRn are broken down instead of recycled back into the body.
Next, and you might have guessed it already, lower levels of IgG mean lower levels of harmful IgG targeting AChR or MuSK receptors on muscle cells. This can lead to improvement in gMG symptoms.
IMAAVY™ is designed to reduce harmful IgG antibodies, including anti-AChR and anti-MuSK, by binding and blocking FcRn receptors. As we have learned, these harmful IgG antibodies can cause gMG symptoms. With IMAAVY™ binding and blocking FcRn receptors, there are lower amounts of harmful IgG, including anti-AChR and anti-MuSK, which can result in fewer interruptions between the nerves and muscles.
And fewer interruptions between nerves and muscles may mean that daily activities affected by gMG can become easier for you. In a clinical study, the most common side effects with IMAAVY™ included infection in parts of your body that you use for breathing (known as respiratory tract infection), swelling in your hands, ankles, or feet (known as peripheral edema), and muscle spasms.
So, if you’re looking for a treatment to help manage your gMG symptoms, ask your doctor about IMAAVY™ today!
What is IMAAVY™ (nipocalimab)?
IMAAVY™ is a prescription medicine used to treat adults and children 12 years of age and older with a disease called generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive.
It is not known if IMAAVY™ is safe and effective in children under 12 years of age.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about IMAAVY™?
IMAAVY™ is a prescription medicine that may cause serious side effects, including:
Do not receive IMAAVY™ if you have a severe allergic reaction to nipocalimab or any of the ingredients in IMAAVY™. Reactions have included angioedema and anaphylaxis.
Before using IMAAVY™ tell your healthcare provider about all of your medical conditions, including if you:
Pregnancy Safety Study. There is a pregnancy safety study for IMAAVY™ if IMAAVY™ is given during pregnancy or you become pregnant while receiving IMAAVY™. Your healthcare provider should report IMAAVY™ exposure by contacting Janssen at 1-800-526-7736 or www.IMAAVY.com.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of IMAAVY™?
IMAAVY™ may cause serious side effects. See “What is the most important information I should know about IMAAVY™?”
The most common side effects of IMAAVY™ include: respiratory tract infection, peripheral edema (swelling in your hands, ankles, or feet), and muscle spasms.
These are not all the possible side effects of IMAAVY™. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see the full Prescribing Information and Medication Guide for IMAAVY™ and discuss any questions you have with your doctor.
Dosage Form and Strengths: IMAAVY™ is supplied as a 300 mg/1.62 mL and a 1,200 mg/6.5 mL (185 mg/mL) single-dose vial per carton for intravenous injection.




IMAAVY withMe is here for you and your loved ones with free
personalized support when and where you may need it.
Not ready to sign up yet?
